ADVERSE REACTIONS IN ADOLESCENTS*1
(≥2% in the ZOMIG Nasal Spray groups and > placebo)
(≥2% in the ZOMIG Nasal Spray groups and > placebo)
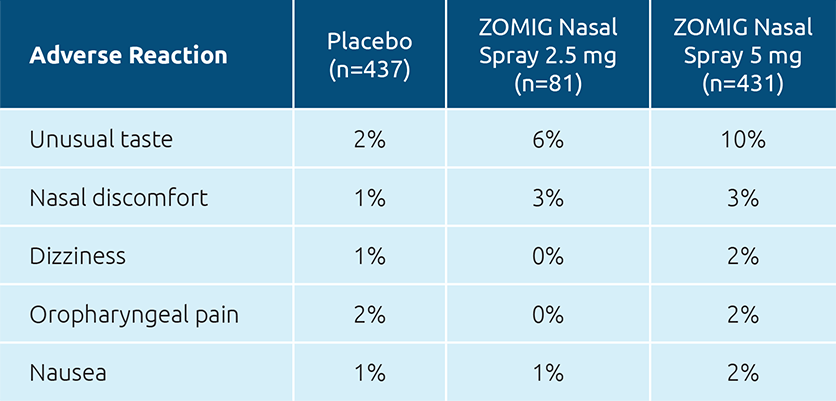
*The safety of ZOMIG Nasal Spray in the acute treatment of migraine in adolescent patients ages 12 to 17 years was established in 2 placebo-controlled studies.1
- The adverse reaction profile was similar across both genders1
- Safety and effectiveness of ZOMIG Nasal Spray in pediatric patients younger than 12 years of age have not been established1
*The safety of ZOMIG Nasal Spray in the acute treatment of migraine in adolescent patients ages 12 to 17 years was established in 2 placebo-controlled studies.1
ZOMIG Nasal Spray is a serotonin (5-HT)1B/1D receptor agonist (triptan) indicated for the acute treatment of migraine with or without aura in adults and pediatric patients 12 years and older.
Limitations of Use:
Use ZOMIG Nasal Spray only after a clear diagnosis of migraine has been established. If a patient has no response to ZOMIG Nasal Spray treatment for the first migraine attack, reconsider the diagnosis of migraine before ZOMIG Nasal Spray is administered to treat any subsequent attacks. ZOMIG Nasal Spray is not indicated for the prevention of migraine attacks. Safety and effectiveness of ZOMIG Nasal Spray have not been established for cluster headache. ZOMIG Nasal Spray is not recommended in patients with moderate to severe hepatic impairment.
Important Safety Information
Contraindications:
- History of coronary artery disease (CAD) or coronary artery vasospasm or other significant underlying cardiovascular disease
- Wolff-Parkinson-White Syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders
- History of stroke, transient ischemic attack, or hemiplegic or basilar migraine
- Peripheral vascular disease
- Ischemic bowel disease
- Uncontrolled hypertension
- Recent (within 24 hours) use of another 5-HT1 agonist (eg, another triptan), or ergot-type medication
- Current or recent (past 2 weeks) use of monoamine oxidase (MAO)-A inhibitor
- Known hypersensitivity to ZOMIG, ZOMIG-ZMT, or ZOMIG Nasal Spray
Warnings and Precautions:
- Myocardial ischemia, myocardial infarction, and Prinzmetal’s Angina: Perform a cardiovascular evaluation in triptan-naïve patients who have multiple cardiovascular risk factors and if satisfactory, consider administrating the first ZOMIG Nasal Spray dose in a medically supervised setting
- Arrhythmias: Discontinue ZOMIG Nasal Spray if these occur
- Sensations of tightness, pain, pressure in the chest, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with 5-HT1 agonists like ZOMIG Nasal Spray and are usually non-cardiac in origin. Perform a cardiac evaluation if these patients are at cardiac risk
- Cerebrovascular events: Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, some resulting in fatalities. Discontinue ZOMIG Nasal Spray if any of these events occur
- ZOMIG Nasal Spray may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction, splenic infarction, and Raynaud’s syndrome. Discontinue ZOMIG Nasal Spray if any of these events occur
- Transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists
- Overuse of acute migraine drugs may lead to exacerbation of headache. Detoxification may be necessary
- Serotonin syndrome may occur with triptans, including ZOMIG Nasal Spray, particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, and MAO inhibitors. Discontinue ZOMIG Nasal Spray if serotonin syndrome is suspected
- Increase in blood pressure
Adverse Reactions
- Adults: unusual taste, paresthesia, dizziness, and hyperesthesia
- Pediatrics: unusual taste
Drug Interactions
- Cimetidine: If co-administered, limit the maximum single dose of ZOMIG Nasal Spray to 2.5 mg, not to exceed 5 mg in any 24-hour period
Use in Specific Populations
- Pregnancy: Based on animal data, ZOMIG Nasal Spray may cause fetal harm
- Lactation: There are no data on the presence of zolmitriptan or its metabolites in human milk, effects on milk production, or on the breastfed infant
- Pediatrics: Safety and effectiveness of ZOMIG Nasal Spray in patients <12 years of age have not been established
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Specialty, a division of Amneal Pharmaceuticals LLC at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
