STRAIGHTFORWARD ADMINISTRATION1
How to use ZOMIG Nasal Spray
- Blow nose gently before use
- Remove the protective cap

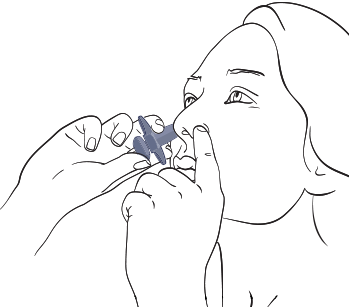
- Hold device as shown
 With the head upright, gently close 1 nostril with the index finger and breathe out through the mouth
With the head upright, gently close 1 nostril with the index finger and breathe out through the mouth
- Put the tip of the sprayer device into the other nostril as far as feels comfortable, and tilt the head back slightly
- Breathe in slowly and gently through the nose, and, at the same time, press the plunger firmly with the thumb
- Keep the head slightly tilted back, remove the tip from the nose, and breathe gently through the mouth for 5 to 10 seconds
Note: There is only 1 dose in the nasal sprayer. Patients should not try to prime the nasal sprayer or press the plunger until they have put the tip into the nostril, or they will lose the dose.
Keep out of reach of children. Store at room temperature between 68°F to 77°F (20°C–25°C). Properly dispose of the ZOMIG Nasal Spray device after completing the full dose or as soon as it becomes outdated or is no longer needed. Do not reuse.
ZOMIG Nasal Spray is a serotonin (5-HT)1B/1D receptor agonist (triptan) indicated for the acute treatment of migraine with or without aura in adults and pediatric patients 12 years and older.
Limitations of Use:
Use ZOMIG Nasal Spray only after a clear diagnosis of migraine has been established. If a patient has no response to ZOMIG Nasal Spray treatment for the first migraine attack, reconsider the diagnosis of migraine before ZOMIG Nasal Spray is administered to treat any subsequent attacks. ZOMIG Nasal Spray is not indicated for the prevention of migraine attacks. Safety and effectiveness of ZOMIG Nasal Spray have not been established for cluster headache. ZOMIG Nasal Spray is not recommended in patients with moderate to severe hepatic impairment.
Important Safety Information
Contraindications:
- History of coronary artery disease (CAD) or coronary artery vasospasm or other significant underlying cardiovascular disease
- Wolff-Parkinson-White Syndrome or arrhythmias associated with other cardiac accessory conduction pathway disorders
- History of stroke, transient ischemic attack, or hemiplegic or basilar migraine
- Peripheral vascular disease
- Ischemic bowel disease
- Uncontrolled hypertension
- Recent (within 24 hours) use of another 5-HT1 agonist (eg, another triptan), or ergot-type medication
- Current or recent (past 2 weeks) use of monoamine oxidase (MAO)-A inhibitor
- Known hypersensitivity to ZOMIG, ZOMIG-ZMT, or ZOMIG Nasal Spray
Warnings and Precautions:
- Myocardial ischemia, myocardial infarction, and Prinzmetal’s Angina: Perform a cardiovascular evaluation in triptan-naïve patients who have multiple cardiovascular risk factors and if satisfactory, consider administrating the first ZOMIG Nasal Spray dose in a medically supervised setting
- Arrhythmias: Discontinue ZOMIG Nasal Spray if these occur
- Sensations of tightness, pain, pressure in the chest, and heaviness in the precordium, throat, neck, and jaw commonly occur after treatment with 5-HT1 agonists like ZOMIG Nasal Spray and are usually non-cardiac in origin. Perform a cardiac evaluation if these patients are at cardiac risk
- Cerebrovascular events: Cerebral hemorrhage, subarachnoid hemorrhage, and stroke have occurred in patients treated with 5-HT1 agonists, some resulting in fatalities. Discontinue ZOMIG Nasal Spray if any of these events occur
- ZOMIG Nasal Spray may cause non-coronary vasospastic reactions, such as peripheral vascular ischemia, gastrointestinal vascular ischemia and infarction, splenic infarction, and Raynaud’s syndrome. Discontinue ZOMIG Nasal Spray if any of these events occur
- Transient and permanent blindness and significant partial vision loss have been reported with the use of 5-HT1 agonists
- Overuse of acute migraine drugs may lead to exacerbation of headache. Detoxification may be necessary
- Serotonin syndrome may occur with triptans, including ZOMIG Nasal Spray, particularly during co-administration with selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants, and MAO inhibitors. Discontinue ZOMIG Nasal Spray if serotonin syndrome is suspected
- Increase in blood pressure
Adverse Reactions
- Adults: unusual taste, paresthesia, dizziness, and hyperesthesia
- Pediatrics: unusual taste
Drug Interactions
- Cimetidine: If co-administered, limit the maximum single dose of ZOMIG Nasal Spray to 2.5 mg, not to exceed 5 mg in any 24-hour period
Use in Specific Populations
- Pregnancy: Based on animal data, ZOMIG Nasal Spray may cause fetal harm
- Lactation: There are no data on the presence of zolmitriptan or its metabolites in human milk, effects on milk production, or on the breastfed infant
- Pediatrics: Safety and effectiveness of ZOMIG Nasal Spray in patients <12 years of age have not been established
To report SUSPECTED ADVERSE REACTIONS, contact Amneal Specialty, a division of Amneal Pharmaceuticals LLC at 1-877-835-5472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
